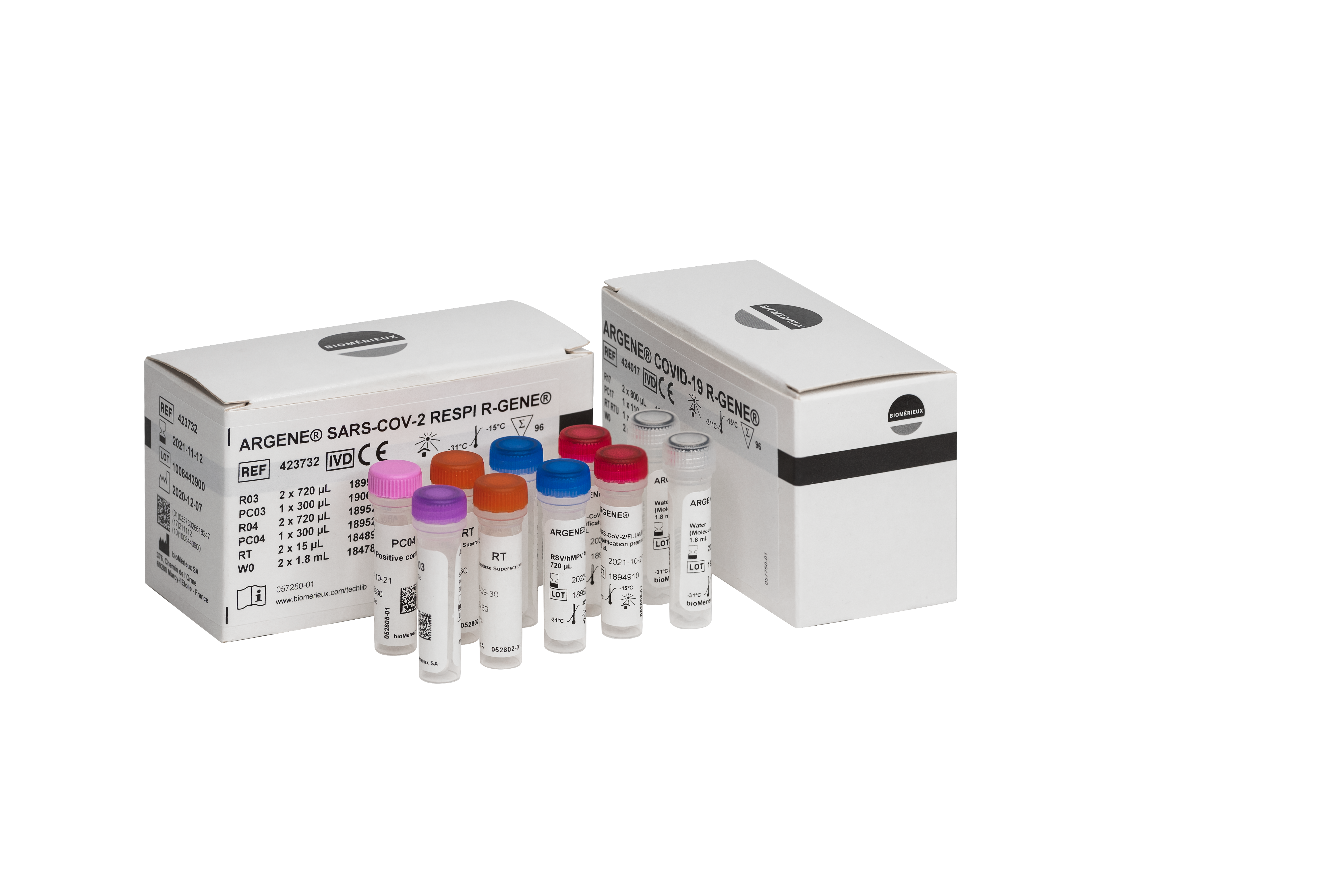
COVID-19 R-GENE® (424017) – Real Time Detection kit
COVID-19 R-GENE® advantages
- Reliable: Highly preserved targeted genes insuring SARS-CoV-2 wild & variants detection
- Secured results: Endogenous internal control to check for extraction/amplification process and sample quality
- Optimized workflow: Shorter amplification run time
- Easy-to-use: All-in-one kit validated for use on the most common extraction and amplification platforms
- Laboratory workflow integration: For use on manual workflow or on automated ARGENE® SOLUTION workflow
Everything you need in one kit
Assay includes all necessary reagents to detect SARS-CoV-2:
- Ready-to use master mix (including Taq polymerase)
- Ready-to-use reverse transcriptase
- Positive control
- Negative control
Easy procedure
Using COVID-19 R-GENE® assay is easy.
| Weight | 1 kg |
|---|---|
| Certification | CE-IVD |
| Incoterms | EXW |
$7.00
| Principle of the test | Genomic detection of SARS-CoV-2 |
| Ordering information | Reference: 424017Designation: COVID-19 R-GENE® - Real Time Detection kit |
| Technology | Real-Time PCR / 5’ nuclease technology |
| PCR design & Gene target |
|
| Specimen |
|
| Controls included | Negative Control, Positive Control, Endogenous Internal Control |
| Number of tests | 96 tests |
| Storage conditions | -15°C/-31°C |
| Extraction platform |
|
| Amplification platform |
|
| Status | For In vitro Diagnostic use (CE-IVD) |
Additional information
| Weight | 1 kg |
|---|---|
| Certification | CE-IVD |
| Incoterms | EXW |
Vendor Information
- Store Name: bioMérieux
- Vendor: Lalla Haidara
- Address: 376, chemin de l'Orme 69280 Marcy l'Etoile
69280 LYON
France - No ratings found yet!