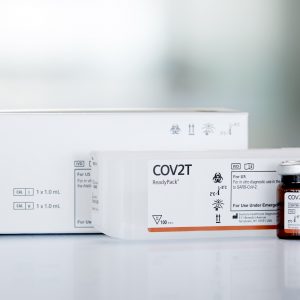
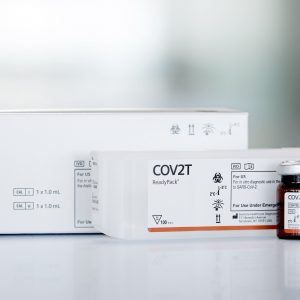
DIAGNOVITAL SARS-CoV-2 (IVD) 100T
- In vitro nucleic acid amplification assay for qualitative detection of 2019-Novel Coronavirus (SARS-CoV-2) in respiratory specimens &Pan-Sarbecovirus
- Detects SARS-CoV like viruses
| Weight | 0.06 kg |
|---|---|
| Brand | Siemens Healthineers |
| Certifications | CE, EUFDA, IVD |
| Incoterms | EXW |
$1,200.00
50000 in stock
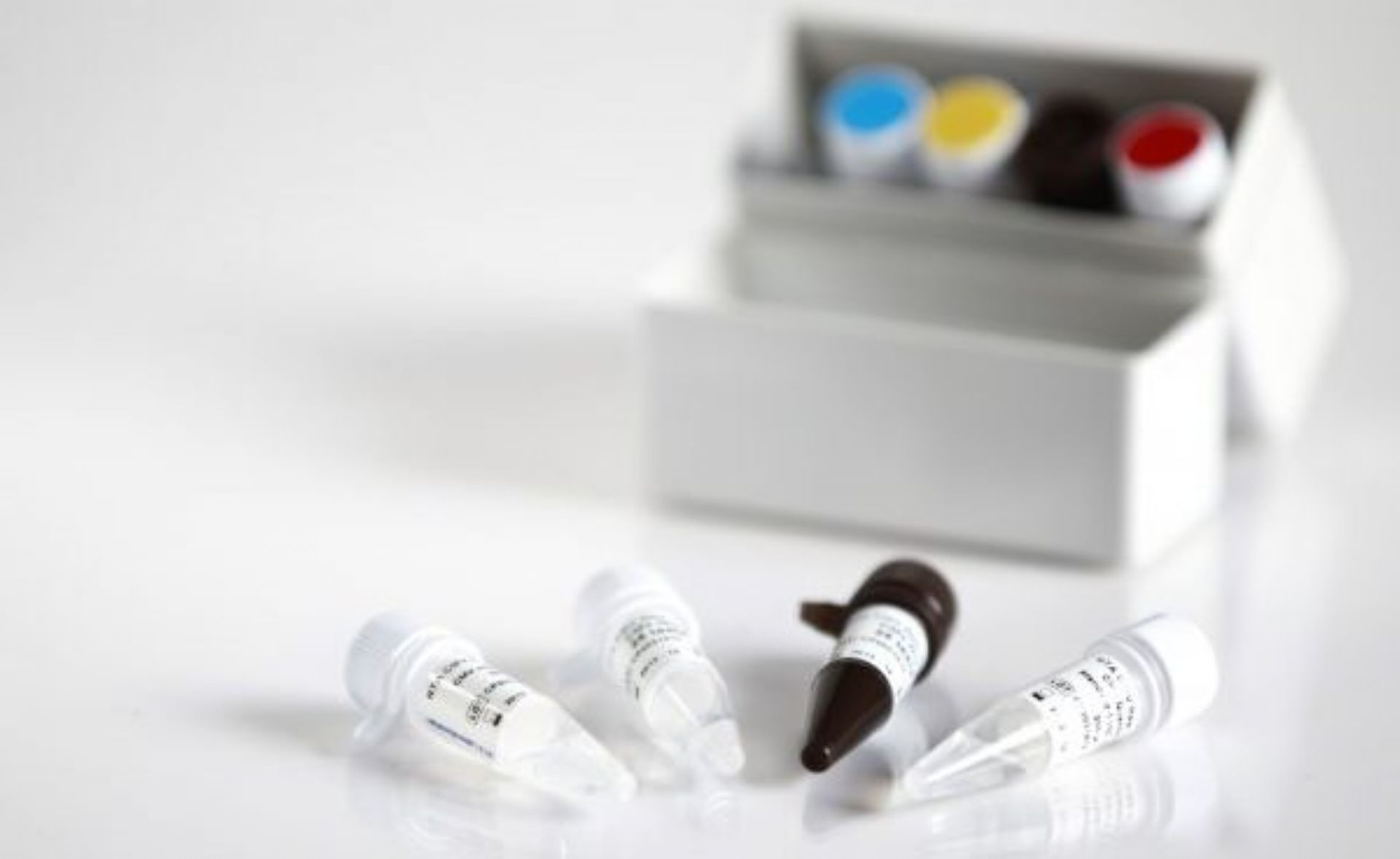
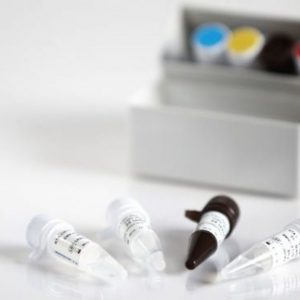
The DIAGNOVITAL SARS-CoV-2 Real-Time PCR Kit has a classic and proven assay design that targets both the RdRP gene and E-gene for the detection of SARS-CoV-2 and Sarbecoviruses, respectively.This product provides additional confidence around specimen integrity through the inclusion of a Human RNaseP target as an extraction control. A broad range of extraction and thermal cyclers have been validated or shown to be compatible in order to provide maximal workflow flexibility for the lab.
Additional information
| Weight | 0.06 kg |
|---|---|
| Brand | Siemens Healthineers |
| Certifications | CE, EUFDA, IVD |
| Incoterms | EXW |
Only logged in customers who have purchased this product may leave a review.
Vendor Information
- Store Name: Siemens Healthineers
- Vendor: Alfred Mawela
- Address: Building 40, Dubai Healthcare City P.O. Box 125936 Dubai, United Arab Emirates
- No ratings found yet!


Reviews
There are no reviews yet.