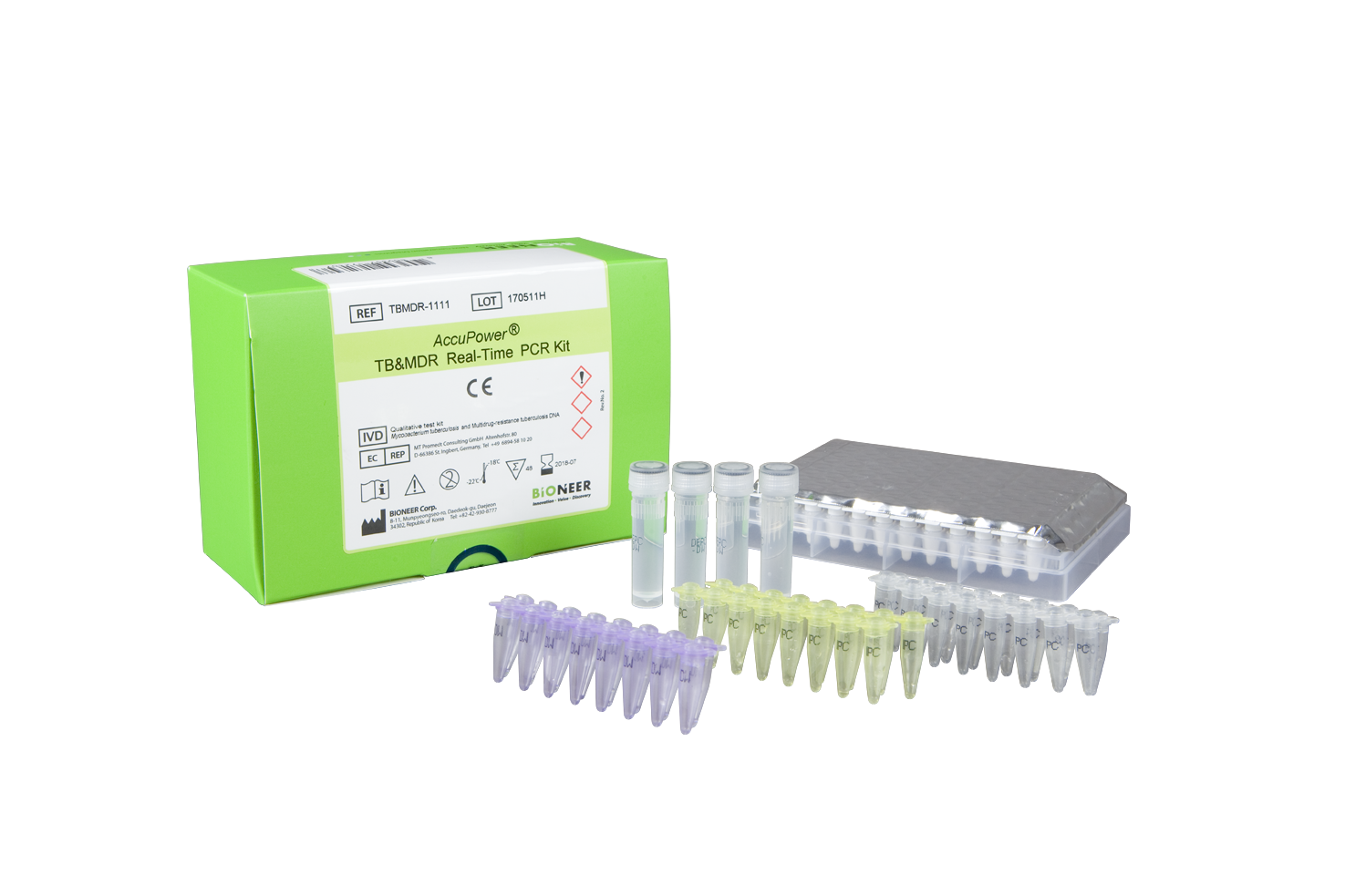
AccuPower® TB&MDR Real-Time PCR Kit is an in vitro diagnostic kit designed for the simultaneous detection of Mycobacterium tuberculosis (MTB) and muti-drug resistant (MDR) TB DNA in human samples such as sputum, bronchoalveolar lavage (BAL) and urine through real-time PCR.
| Weight | 0.5 kg |
|---|---|
| Certification | CE |
| Incoterms | EXW |
$600.00
Multidrug-resistant TB (MDR-TB) is caused by an organism that is resistant to at least isoniazid (INH) and rifampicin (RIF), the two most potent TB drugs. These drugs are used to treat all persons with TB disease. Spontaneous mutations in the M. tuberculosis genome can give rise to proteins that make the bacterium drug resistant, depending on the drug action. Almost 2~3% of new TB patients have MDR-TB. For the effective treatment of TB and MDR-TB, early detection is the top priority.
Features and Benefits
- Signature Convenience: Multiplex detection of TB and MDR-TB (13 mutant sites, 36 mutations). Simplified procedure of sputum pretreatment with EZ Solution.
- Enhanced Sensitivity & Specificity: Bioneer’s proprietary HotStart technology accomplishes high sensitivity and specificity.
- Remarkable Stability: Vacuum-dried premix allows stable and reproducible results.
- High Quality: All diagnostic kits manufactured by Bioneer are under strict quality control guidelines.
- Sputum Pre-treatment: EZ Solution vs NaOH
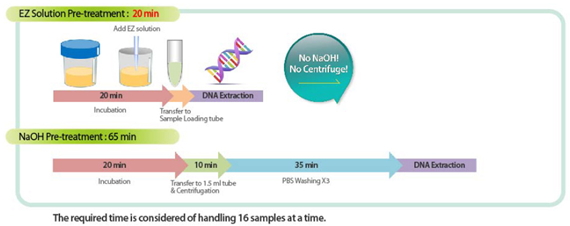
Specifications
| Specimen Type | Sputum, bronchoalveolar lavage (BAL), culture specimen |
| Kit Contents | PCR Premix (RIF/TB&INH), PC, IPC, NTC, DEPC D.W. |
| Instrumentation | ExiStation™ ™ Universal Molecular Diagnostic System or Exicycler™ 96 |
| Tests | 48 |
Additional information
| Weight | 0.5 kg |
|---|---|
| Certification | CE |
| Incoterms | EXW |
Only logged in customers who have purchased this product may leave a review.
Vendor Information
- Store Name: BIONEER Corporation
- Vendor: BIONEER Corporation
-
Address:
8-11, Munpyeongseo-ro
Daejeon
34302
South Korea - No ratings found yet!




Reviews
There are no reviews yet.