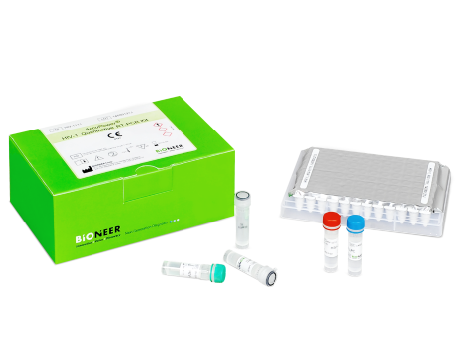
AccuPower® HIV Quantitative RT-PCR Kit is an in vitro diagnostic kit designed for the quantification of HIV-1 RNA in human samples such as serum and EDTA-plasma through real-time PCR.
| Weight | 0.5 kg |
|---|---|
| Certification | CE |
| Incoterms | EXW |
$1,000.00
Human immunodeficiency virus (HIV) is recognized as a known viral agent that causes Acquired Immune Deficiency Syndrome (AIDS). Thus, monitoring of viral load in HIV-1 infection patients is an essential test for establishment of therapeutic strategy and determination of therapeutic progress. A RT (Reverse transcriptase) PCR test is used to measure the amount of HIV RNA. Because this test looks for HIV directly in a person's blood instead of detecting antibodies, it is used by researchers and health care providers to identify infections during the window period.
Features and Benefits
- HIV-1 Subtype Detection: HIV-1 subtype group M (A, B, C, D, AE, F, AG, G, H and several CRFs), N and O are detectable with high sensitivity.
- Signature Convenience: One-step RT-PCR premix type. All components for the assay are contained within a tube.
- Enhanced Sensitivity & Specificity: Bioneer’s proprietary Dual HotStart technology accomplishes high sensitivity and specificity.
- Remarkable Stability: Vacuum-dried premix allows stable and reproducible results
- High Quality: All diagnostic kits manufactured by Bioneer are under strict quality control guidelines
Specifications
| Specimen Type | EDTA-plasma |
| Kit Contents | PCR Premix, SPC 1~5, LPC, HPC, NTC, SL buffer, DEPC D.W. |
| Instrumentation | ExiStation™ Universal Molecular Diagnostic System |
| Tests | 96 |
Additional information
| Weight | 0.5 kg |
|---|---|
| Certification | CE |
| Incoterms | EXW |
Only logged in customers who have purchased this product may leave a review.
Vendor Information
- Store Name: BIONEER Corporation
- Vendor: BIONEER Corporation
-
Address:
8-11, Munpyeongseo-ro
Daejeon
34302
South Korea - No ratings found yet!



Reviews
There are no reviews yet.