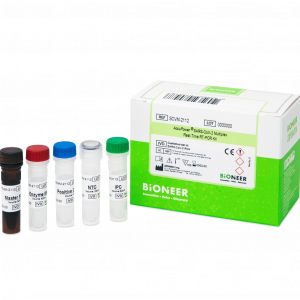
AccuPower® STI8B-Plex Real-Time PCR Kit is an in vitro diagnostic kit designed for the simultaneous detection of Trichomonas vaginalis, Mycoplasma hominis, Herpes simplex virus 1 and 2 DNA in human samples such as urine and vaginal swab through real-time PCR.
| Weight | 0.5 kg |
|---|---|
| Certification | CE |
| Incoterms | EXW |
$800.00
Trichomoniasis is one of the most common STIs caused by a small organism called Trichomonas vaginalis. Women are most often affected by this disease, although men can become infected and pass the infection to their partners through sexual contact. Mycoplasma hominis is a strain of bacteria that is present in the urogenital area. Infection is usually asymptomatic, but for pregnant women, infection can lead to premature birth. Herpes simplex type 1, which is transmitted through oral secretions or sores on the skin, can be spread through kissing or sharing objects such as toothbrushes or eating utensils.
In general, a person can only get herpes type 2 infections during sexual contact with someone who has a genital HSV-2 infection. Depending on the disease, some untreated STIs can lead to infertility, chronic pain or even death. Early identification and treatment results in less chance to spread disease and for some conditions may improve the outcomes of treatment.
Features and Benefits
- Multiplex Detection: Simultaneous detection of 8 STI pathogens with STI8A-Plex Real-Time PCR Kit using ExiStation™ MDx System
- Signature Convenience: All components for the assay are contained within a tube.
- Enhanced Sensitivity & Specificity: Bioneer’s proprietary HotStart technology accomplishes high sensitivity and specificity.
- Remarkable Stability: Vacuum-dried premix allows stable and reproducible results.
- High Quality: All diagnostic kits manufactured by Bioneer are under strict quality control guidelines.
Specifications
| Specimen Type | Urine, vaginal swab, urethral swab |
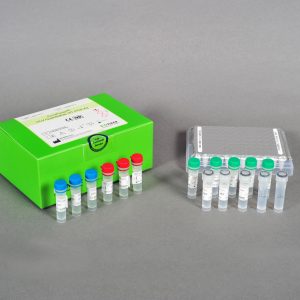
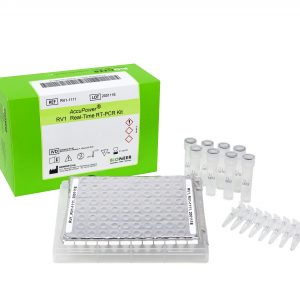
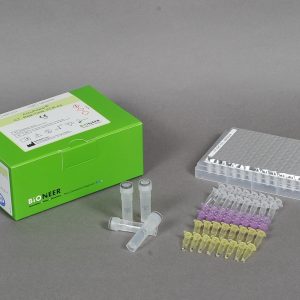
| Kit Contents | PCR Premix, PC, IPC, NTC, DEPC D.W. |
| Instrumentation | ExiStation™ Universal Molecular Diagnostic System or Exicycler™ 96 |
| Tests | 96 |
Additional information
| Weight | 0.5 kg |
|---|---|
| Certification | CE |
| Incoterms | EXW |
Only logged in customers who have purchased this product may leave a review.
Vendor Information
- Store Name: BIONEER Corporation
- Vendor: BIONEER Corporation
- Address: 8-11, Munpyeongseo-ro
Daejeon
34302
South Korea - No ratings found yet!



Reviews
There are no reviews yet.