- NMPA Certified factory, CE certified
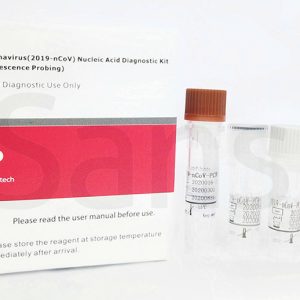
- Detection Kit for 2019 Novel Coronavirus (2019-nCoV) RNA (PCR- Fluorescence Probing)
- RNA extraction or purification reagents
- Viral transport medium and sampling collection swabs (individually wrapped)
- 96tests/kit
| Minimum Order | 10000 Tests |
|---|---|
| Certification | CE, NMPA, WHO EUL |
$3.80
4553808 in stock
This kit is used for the in vitro qualitative detection of novel coronavirus (2019-nCoV) ORFlab and N gene in the throat swabs, sputum specimens of suspected pneumonia patients infected by a novel coronavirus, suspected clustering cases, and others needing a diagnosis or differential diagnosis for novel coronavirus. For the definitions of "suspected cases" and "suspected clustering cases", refer to the documents(current versions) such as Diagnosis and Treatment Scheme for Pneumonia Patients Infected by NovelCoronavirus and Monitoring Scheme for Pneumonia Patients Infected by Novel Coronavirus issued by the Chinese Center for Disease Control and Prevention (China CDC). This product is only used for the auxiliary diagnosis and in vitro diagnosis (IVD) of pneumonia infected by a novel coronavirus (2019-nCoV) since December 2019 under emergency. Instead, it cannot be clinically used as a general IVD reagent. In use, the relevant requirements of the documents such as Diagnosis and Treatment Scheme for Pneumonia Patients Infected by Novel Coronavirus and Monitoring Scheme for Pneumonia Patients Infected by Novel Coronavirus shall be followed. Detection of novel coronavirus RNA shall meet the requirements of the Technical Guideline of the China CDC for Laboratory Detection of New Coronavirus Pneumonia and other documents, so as to carry out the regulations on biosafety. The detection results of this kit are for clinical reference only and should not be used as the sole criteria for clinical diagnosis. It is recommended to conduct a comprehensive analysis of the condition in combination with the clinical manifestations of the patient and other laboratory tests.
[Storage Conditions and Validity Date] The kit is stored at -20±5℃, and the validity period is 12 months. The reagent can be stored at 4°C for 3 days; the kit can be stored at 37°C for 3 days; repeated freezing and thawing should be avoided; repeated freezing and thawing cycles should not exceed 7 times and the times of decapping the reagent should not exceed 7.
Useful Link: Click here
Additional information
| Minimum Order | 10000 Tests |
|---|---|
| Certification | CE, NMPA, WHO EUL |
Only logged in customers who have purchased this product may leave a review.
Vendor Information
- Store Name: AMSP LTD
- Vendor: AMSP LTD
- Address:
- No ratings found yet!





Reviews
There are no reviews yet.