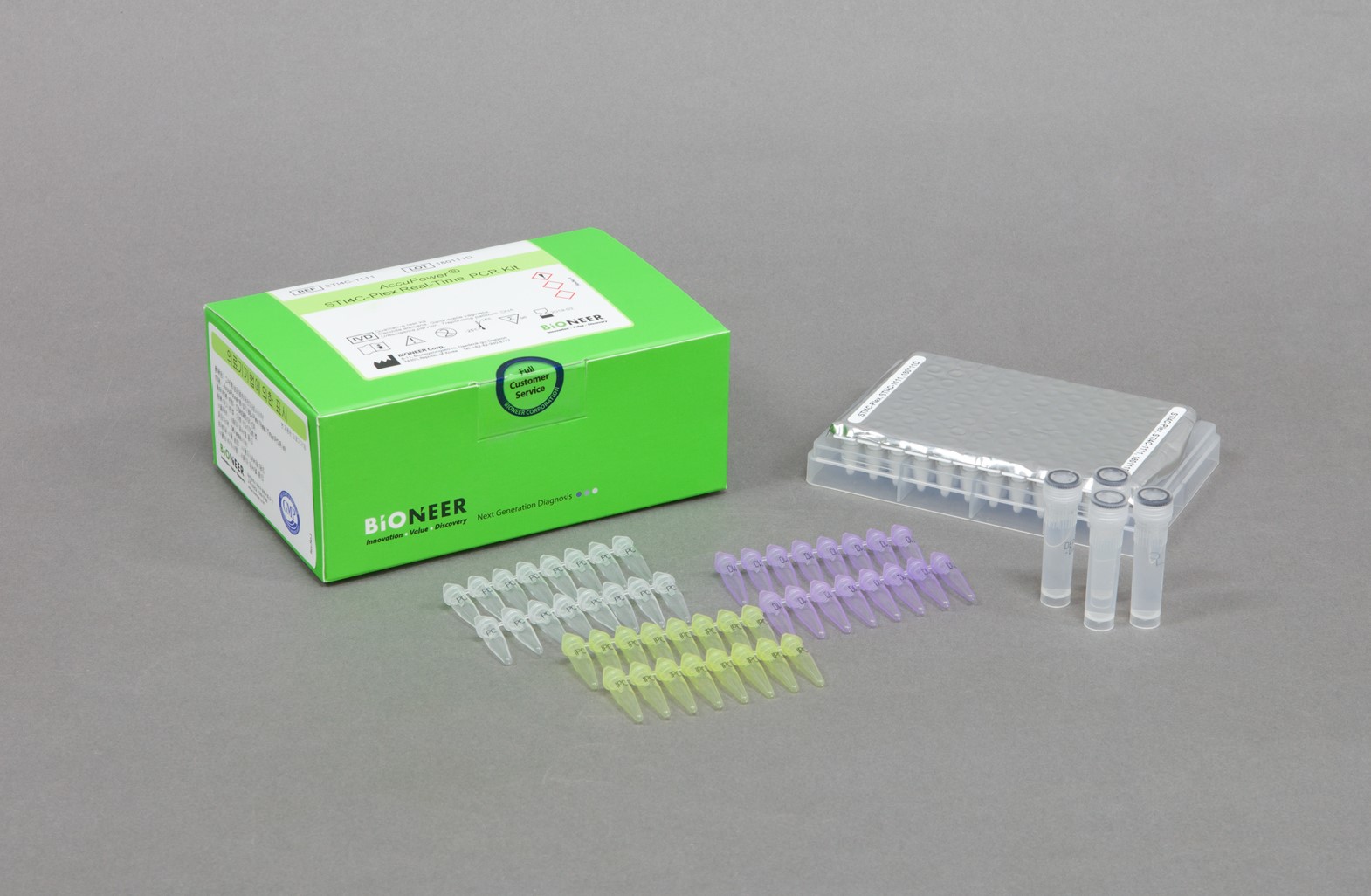
The AccuPower® STI4C-Plex Real-Time PCR Kit is an in vitro diagnostic kit designed for the simultaneous detection of Candida albicans, Gardnerella vaginalis, Ureaplasma parvum and Treponema pallidum DNA in human samples such as urine and vaginal swab through real-time PCR using ExiStation™ Molecular Diagnostic System or Exicycler™ 96
| Weight | 0.5 kg |
|---|---|
| Certification | CE |
| Incoterms | EXW |
$800.00
Sexually Transmitted Infection (STI) is an infection that can be transferred from one person to another by means of sexual contact, including oral sex, anal sex, and sharing sex toys. Candida albicans (CA) cause common vaginal yeast infection, which 75 percent of women experience once in their lifetime. Symptoms include itching of the external genitals and pain and discomfort during urination. Gardnerella vaginalis (GV), like Candida albicans, is the most common cause of women's vaginitis, pelvic inflammatory disease and endometriosis in women. Ureaplasma can be divided into Ureaplasma urealyticum (UU) and Ureaplasma parvum (UP). Ureaplasma parvum (UP) is less pathogenic than other pathogen unlike other sexually transmitted bacteria. It can be pathogenic to both men and women, and can cause uteritis in pregnant women. Finally, Treponema pallidum (TP) is a bacterium that causes syphilis. In adults, although the mildew is a characteristic lesion, it can affect skin ulcers, paralysis and organ damage depending on the extent. In the latter part of pregnancy, pathogens can spread to the fetus and endanger the life of the fetus.Early identification and treatment results in less chance to spread disease and for some conditions may improve the outcomes of treatment.
Features and Benefits
- Multiplex Detection: Simultaneous detection of 8 STI pathogens with STI8A-Plex and STI8B-Plex Real-Time PCR Kit using ExiStation™ MDx System.
- Signature Convenience: All components for the assay are contained within a tube.
- Enhanced Sensitivity & Specificity: Bioneer’s proprietary HotStart technology accomplishes high sensitivity and specificity.
- Remarkable Stability: Vacuum-dried premix allows stable and reproducible results.
- High Quality: All diagnostic kits manufactured by Bioneer are under strict quality control guidelines.
Specifications
| Specimen Type | Urine, vaginal swab |
| Kit Contents | PCR Premix, PC, IPC, NTC, DEPC D.W. |
| Instrumentation | ExiStation™ Universal Molecular Diagnostic System or Exicycler™ 96 |
| Tests | 96 |
Additional information
| Weight | 0.5 kg |
|---|---|
| Certification | CE |
| Incoterms | EXW |
Only logged in customers who have purchased this product may leave a review.
Vendor Information
- Store Name: BIONEER Corporation
- Vendor: BIONEER Corporation
-
Address:
8-11, Munpyeongseo-ro
Daejeon
34302
South Korea - No ratings found yet!



Reviews
There are no reviews yet.