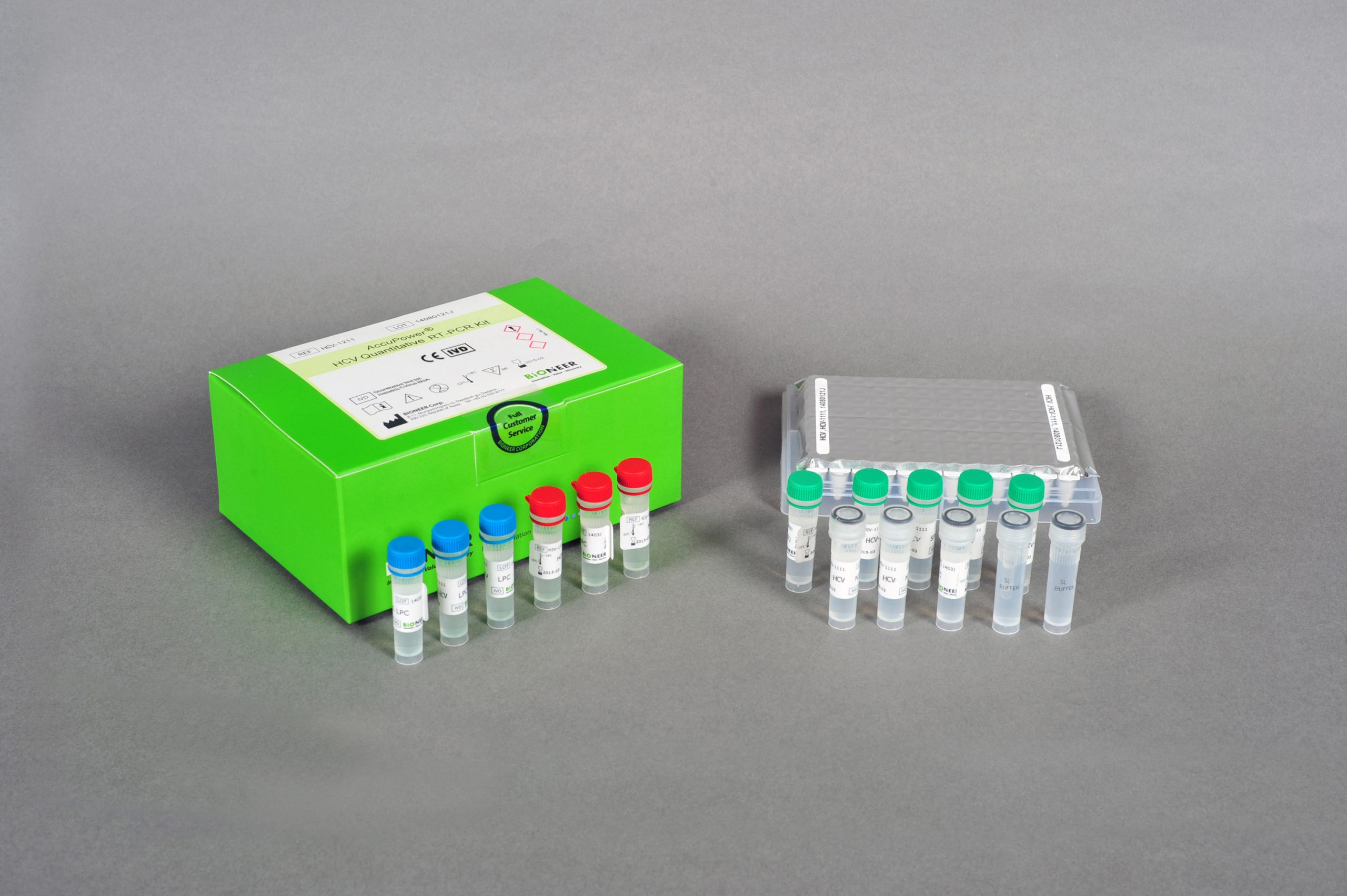
AccuPower® HCV Quantitative RT-PCR Kit is an in vitro diagnostic kit designed for the quantification of hepatitis C virus (HCV) RNA in human samples such as serum and EDTA-plasma through real-time PCR.
| Weight | 0.5 kg |
|---|---|
| Certification | CE |
| Incoterms | EXW |
$750.00
Hepatitis C is a liver disease caused by hepatitis C virus (HCV). HCV infection is generally asymptomatic during the acute phase and about 85% of them become chronically infected. However, most instances of acute infection are clinically undetectable. In rare cases, acute hepatitis is accompanied by jaundice, malaise, weakness and anorexia. Since HCV cannot be grown in the clinical laboratory, more sensitive molecular testing is needed to confirm the presence of the virus such as quantitative real-time PCR.
Features and Benefits
- HCV Genotype Detection: HCV genotype 1, 2, 3, 4, 5 and 6 are detectable with high sensitivity.
- Signature Convenience: One-step RT-PCR premix type. All components for the assay are contained within a tube.
- Enhanced Sensitivity & Specificity: Bioneer’s proprietary Dual HotStart technology accomplishes high sensitivity and specificity.
- Remarkable Stability: Vacuum-dried premix allows stable and reproducible results.
- High Quality: All diagnostic kits manufactured by Bioneer are under strict quality control guidelines.
Specifications
| Specimen Type | Serum, EDTA-plasma |
| Kit Contents | PCR Premix, SPC 1~5, LPC, HPC, NTC, SL buffer, DEPC D.W. |
| Instrumentation | ExiStation™ Universal Molecular Diagnostic System |
| Tests | 96 |
Additional information
| Weight | 0.5 kg |
|---|---|
| Certification | CE |
| Incoterms | EXW |
Only logged in customers who have purchased this product may leave a review.
Vendor Information
- Store Name: BIONEER Corporation
- Vendor: BIONEER Corporation
-
Address:
8-11, Munpyeongseo-ro
Daejeon
34302
South Korea - No ratings found yet!



Reviews
There are no reviews yet.