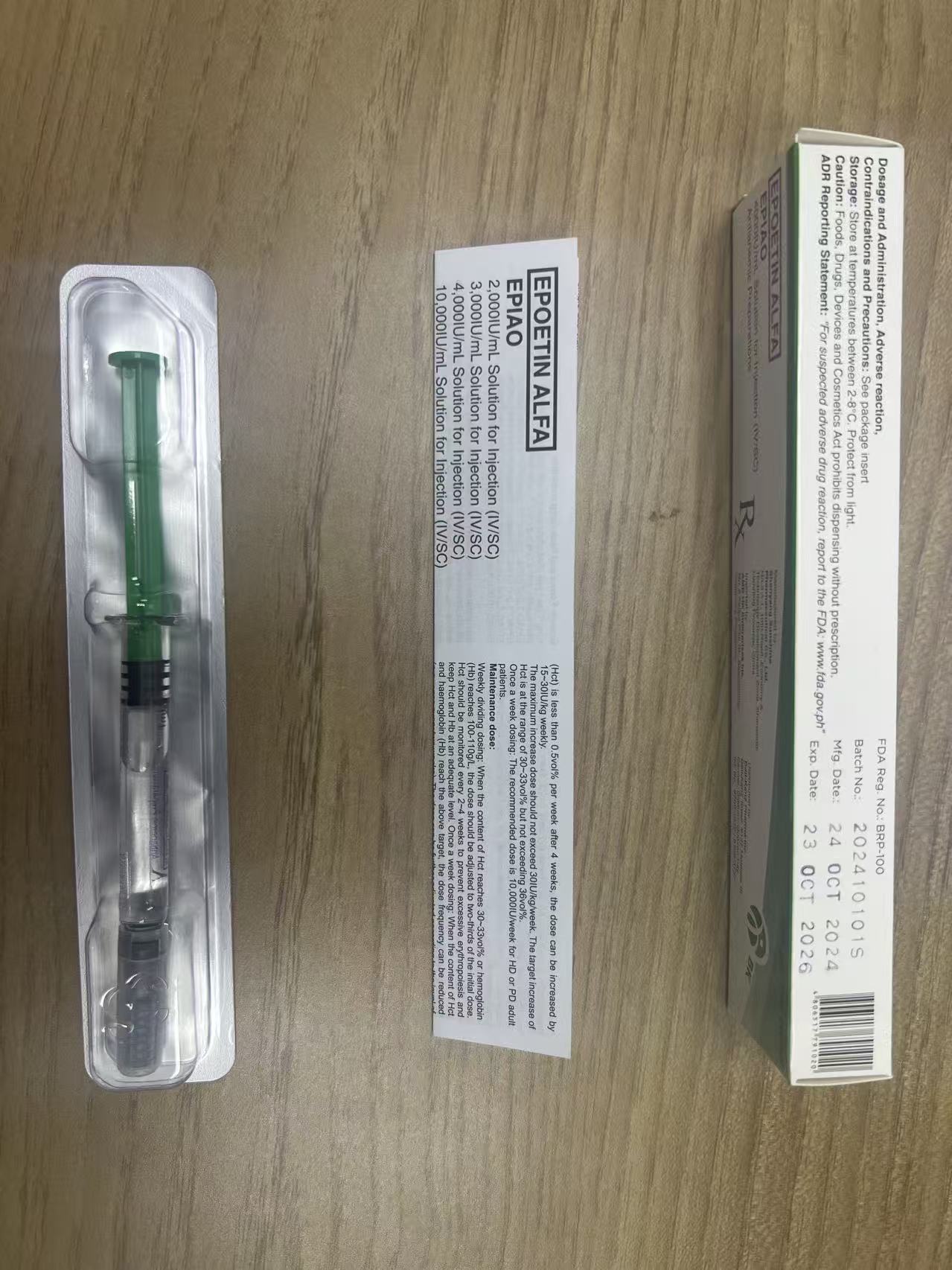
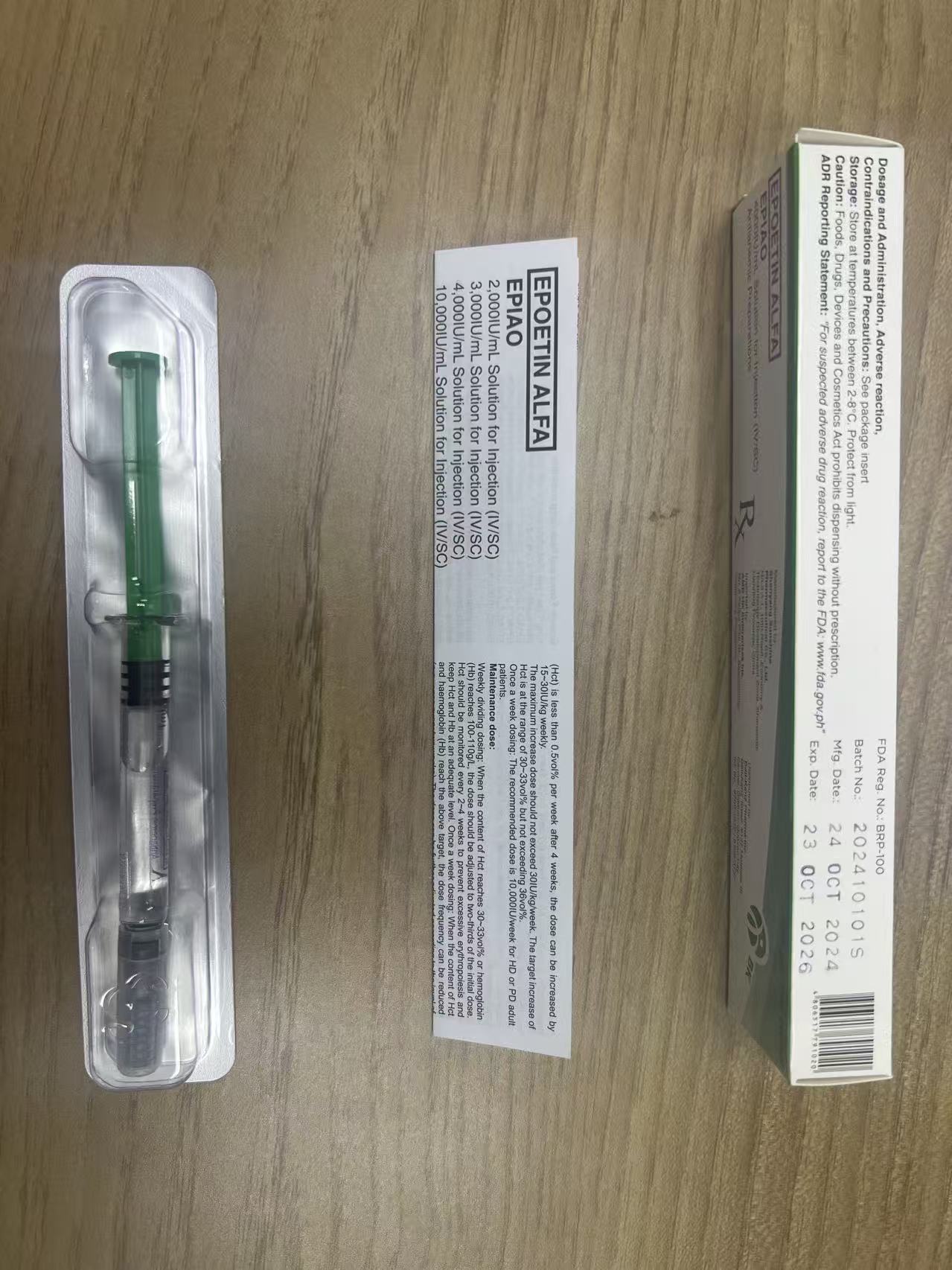
EPlAO is colorless and transparent liquid in pre-filled syringe or single-use vial.Epoetin alfa is recombinant human erythropoietin (EPO). lt is expressed in Chinese hamster ovarycells (CHO) and has a 165 amino acid sequence identical to that of human urinary EPO; the two are indistinguishable on the basis of functional assays. The apparent molecular weight of erythropoietin is about 30,400 daltons.
$2.00
1. EPlAO is indicated for the treatment of symptomatic anaemia associated with chronic renal failure(CRF):
-in adults and paediatrics aged 1 to 18 years on haemodialysis and adult patients on peritoneal dialysis.
-in adults with renal insufficiency not yet undergoing dialysis for the treatment of severe anaemiaof renal origin accompanied by clinical symptoms in patients.
2. EPlAO is indicated in adults receiving chemotherapy for solid tumours, malignant lymphoma ormultiple myeloma, and at risk of transfusion as assessed by the patient's general status (e.g.cardiovascular status, pre-existing anaemia at the start of chemotherapy) for the treatment of anaemiaand reduction of transfusion requirements.
3. EPlAO is indicated in adults in a predonation proaramme to increase the vield of autologous bloodTreatment should only be given to patients with moderate anaemia (haemoglobin concentration rangebetween 10 to 13 a/dL [6.2 to 8.1 mmol/Ll, no iron deficiency) if blood saving procedures are notavailable or insufficient when the scheduled major elective surgery requires a large volume of blood (4or more units of blood for females or 5 or more units for males).
4. EPlAO is indicated for non-iron deficient adults prior to major elective orthopaedic surgery having ahigh perceived risk for transfusion complications to reduce exposure to allogeneic blood transfusions.Use should be restricted to patients with moderate anaemia (e.g. haemoglobin concentration rangebetween 10 to 13 g/dL) who do not have an autologous predonation programme available and withexpected moderate blood loss (900 to 1.800 mL).
5. EPlAO is indicated for the treatment of symptomatic anaemia (haemoglobin concentration of ≤10g/dL) in adults with low- or intermediate-1-risk primary myelodysplastic syndromes (MDS) who havelow serum erythropoietin(<200 mU/mL).
Only logged in customers who have purchased this product may leave a review.
Vendor Information
- Store Name: Sinopharm Fortune Way Company
- Vendor: Sinopharm Fortune Way Company
- Address: China
- No ratings found yet!




Reviews
There are no reviews yet.