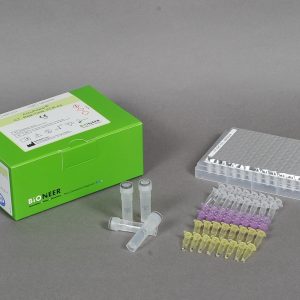
ExiPrep™ 48 Viral DNA/RNA Kit is designed for accurate and rapid extraction of viral DNA or RNA from various human specimens using ExiPrep™ 48 Dx .For HBV and HCV detection, a single sample can be used to detect both targets. The entire extraction process is carried out automatically via ExiPrep™ 48 Dx instrument.
| Weight | 3.25 kg |
|---|---|
| Certification | CE |
| Incoterms | EXW |
$432.00
Features
1. Pre-filled buffer cartridge system
2. Simultaneous extraction of nucleic acids from maximum of 48 clinical samples
3. ExiPrep™48 Dx system uses silica magnetic beads developed and produced with Bioneer’s technology.
Specification
Technology | Magnetic Silica Beads | ||
Main sample type | Body fluids, Swab, Sputum | ||
Starting volume | Serum | 800 ul/400 ul | |
Plasma | |||
Urine | |||
Whole blood | 200 ul | ||
Elution volume | 50 ul | ||
Instrumention | ExiPrep™48 Dx | ||
Test | 96 | ||
Certification | CE, MFDS | ||
Workflow
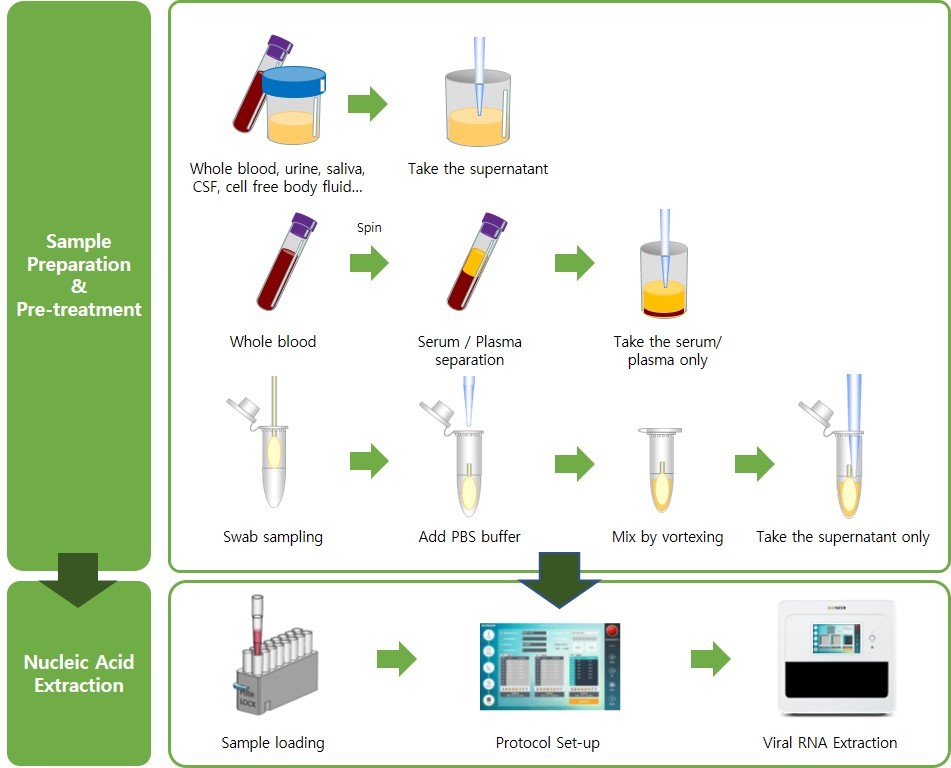
Performances
1.Test results using ExiPrep™ 48 Viral DNA/RNA Kit and AccuPower® HBV Quantitative PCR Kit
HBV | 1.64 Log10 IU/ml (N=9) | 3.33 Log10 IU/ml (N=9) |
Parameter | Log10 IU/ml | Log10 IU/ml |
Avg(range) | 1.81(1.62-1.99) | 3.32(3.18-3.42) |
SD | 0.13 | 0.09 |
%CV | 6.98% | 2.66% |
Bias* | 0.17 | 0.01 |
The efficiency of ExiPrep™ 48 Viral DNA/RNA Kit was confirmed with two quantitative concentrations of HBV panel. Viral DNA was extracted from serum using ExiPrep™ Viral DNA/RNA Kit and real-time PCR was performed with AccuPower® HBV Quantitative PCR Kit.
2. Test results using ExiPrep™ 48 Viral DNA/RNA Kit and AccuPower® HCV Quantitative PCR Kit.
HCV | 1.48 Log10 IU/ml (N=9) | 3 Log10 IU/ml (N=9) |
Parameter | Log10 IU/ml | Log10 IU/ml |
Avg(range) | 1.92(1.71-2.04) | 2.91(2.84-2.97) |
SD | 0.09 | 0.04 |
%CV | 4.90% | 1.48% |
Bias* | 0.44 | 0.09 |
The efficiency of ExiPrep™ 48 Viral DNA/RNA Kit was confirmed with two quantitative concentrations of HCV panel. Viral RNA was extracted from plasma using ExiPrep™ Viral DNA/RNA Kit and real-time PCR was performed with AccuPower® HCV Quantitive PCR Kit.
3. Test results using ExiPrep™ 48 Viral DNA/RNA Kit and AccuPower® CMV Quantitative PCR Kit
CMV | 2.38 Log10 IU/ml (N=20) | 3 Log10 IU/ml (N=20) |
Parameter | Log10 IU/ml | Log10 IU/ml |
Avg(range) | 2.41(2.33-2.55) | 3.00(2.94-3.11) |
SD | 0.07 | 0.05 |
%CV | 2.95% | 1.67% |
Bias* | 0.03 | 0 |
The efficiency of ExiPrep™ 48 Viral DNA/RNA Kit was confirmed with two quantitative concentrations of CMV panel. Viral DNA was extracted from whole blood using ExiPrep™ Viral DNA/RNA Kit and real-time PCR was performed with AccuPower® CMV Quantitative PCR Kit.
Additional information
| Weight | 3.25 kg |
|---|---|
| Certification | CE |
| Incoterms | EXW |
Only logged in customers who have purchased this product may leave a review.
Vendor Information
- Store Name: BIONEER Corporation
- Vendor: BIONEER Corporation
- Address: 8-11, Munpyeongseo-ro
Daejeon
34302
South Korea - No ratings found yet!


Reviews
There are no reviews yet.